名前を赤で表記しているメンバーは当研究室の学生・院生、青で表記しているメンバーは当研究室の教員です。
原著論文
- Shiva Rezaei Motlagh, Ramin Khezri, Mohammad Etesami, Ahmad Azmin Mohamad, Pinit Kidkhunthod, Muhamad Kamil Yaakob, Manaswee Suttipong, Mai Thanh Nguyen, Tetsu Yonezawa, Kasidit Nootong, Soorathep Kheawhom
“Mitigating water-related challenges in aqueous zinc-ion batteries through ether-water hybrid electrolytes”
Electrochimica Acta, 461,143122 (2023). 【Elsevier】(IF = 6.6)
DOI: 10.1016/j.electacta.2023.143122 (Published (web) 1 September 2023)
【国際共同研究】Abstract: Aqueous zinc-ion batteries (AZIBs) are an attractive option for different energy storage applications due to their cost-effectiveness, safety benefits, and high capacity. Nevertheless, their development is constrained by challenges such as hydrogen evolution reaction (HER), significantly affecting the battery lifespan. This work presents a promising solution to this problem using a hybrid electrolyte, consisting of water and tetra ethylene glycol dimethyl ether (TEGDME) containing Zn(OTf)2. Results demonstrate that this hybrid electrolyte can suppress HER and can lessen zinc corrosion by reorienting hydrogen bonds with water and actively participating in the solvation structure of Zn2+ ions. Such reorientation reduces water activity and weakens the interactions between water molecules and between water and Zn2+ ions. By employing an optimal TEGDME/H2O ratio of 2:1 in the hybrid electrolyte, hydrogen evolution overpotential increased by 0.26 V. As a result, in the Zn//Zn symmetrical cells, the developed hybrid electrolyte is seen to enable the Zn electrode to exhibit excellent cycle durability, lasting over 4300 h with coulombic efficiency (CE) of 99.68% at 0.5 mA cm−2 and 0.5 mAh cm−2, surpassing the 92.79% achieved by the base electrolyte (1 M Zn(OTf)2 aqueous solution) cells. Moreover, when implemented in Zn/δ-MnO2 batteries, the hybrid electrolyte maintained its performance for over 1800 cycles at a discharge rate of 100 mA g−1. This cost-effective and adaptable strategy highlights its wide-ranging potential, offering vital insights for the enhancement of AZIB technology.
- Mohammad Etesami, Ramin Khezri, Shiva Rezaei Motlagh, Mohan Gopalakrishnan, Mai Thanh Nguyen, Tetsu Yonezawa, Suttipong Wannapaiboon, Kasidit Nootong, Anongnat Somwangthanaroj, Soorathep Kheawhom
“High-Temperature Air Synthesis: A Facile Approach to Nitrogen-Doped, Metal-Free Carbon Electrocatalysts”
ChemCatChem, 15(18), e202300724 (2023).【Wiley-VCH】(IF=5.501)
DOI: 10.1002/cctc.202300724(Published (web) 5 July 2023)
【国際共同研究】
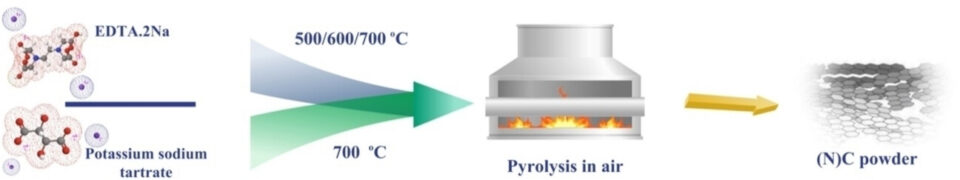
Abstract: This study presents a novel, straightforward method for synthesizing hierarchical nitrogen-doped carbon structures, positioning metal-free, carbon-based materials as potential substitutes in electrochemical reactions such as oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER). The unique method involves a single-step pyrolysis process in an air atmosphere, eliminating the need for an inert atmosphere and pre-treatment procedures. It enables simultaneous self-templating and heteroatom doping, resulting in oxygen-rich functional groups embedded in the nitrogen-doped carbon structure. We also crafted a carbon structure without heteroatom doping, comparing its electrochemical performance in ORR and HER. Our findings indicate that carbon catalysts pyrolyzed at higher temperatures have more pyridinic N, functional groups, and active sites- factors conducive to electrochemical reactions. We tested the air-synthesized electrocatalysts for ORR in alkaline electrolyte and employed the optimized nitrogen-doped carbon catalyst, pyrolyzed at 700 ℃ in an air atmosphere, as cathode material in a zinc-air battery. This catalyst demonstrated ORR performance comparable to the commercial Pt/C catalyst and showed minimal overpotential in acidic HER. Our research establishes a pioneering technique for synthesizing porous, metal-free, nitrogen-doped carbon materials, paving the way for potential energy applications.
- Donghoon Lee, Yohei Ishida, Tetsu Yonezawa
“Unexpected Reactivity of Cationic-to-Cationic Thiolate Ligand-Exchange Reaction on Au25 Clusters”
Langmuir, 39(24), 8435-8440 (2023). 【ACS】(IF = 4.331)
DOI: 10.1021/acs.langmuir.3c00499(Published (web) 7 June 2023)
【研究室内研究】

Abstract: Thiolate-protected molecular noble metal clusters have attracted significant attention due to their unique physicochemical properties, which make them applicable in diverse fields such as catalysis, sensing, and bioimaging. Ligand-exchange reactions are a crucial technique for synthesizing and functionalizing these clusters, as they allow for the introduction of new ligands onto the cluster surface, which can alter their properties. While numerous studies have investigated neutral-to-neutral, neutral-to-anionic, and neutral-to-cationic ligand-exchange reactions, the cationic-to-cationic ligand-exchange reaction has never been reported, making the study of such reactions intriguing. In this study, the cationic ligand-exchange reaction on Au25(4-PyET-CH3+)x(4-PyET)18–x (x ≈ 9) clusters, which contain both neutral and cationic ligands in nearly equivalent amounts, was investigated. Contrary to our expectation that the cationic-to-cationic ligand-exchange reaction would be suppressed due to Coulombic repulsion between the surface cationic ligands and incoming cationic ligands, the originally existing cationic ligand was selectively exchanged. The choice of counterions for cationic ligands played a crucial role in controlling the selectivity of ligand exchange. For instance, bulky and hydrophobic counterions such as PF6– can cause steric hindrance and reduce Coulombic repulsion, which promotes cationic-to-cationic ligand exchange. Conversely, counterions like Cl– can lead to neutral-to-cationic ligand exchange due to reduced steric hindrance and increased Coulombic repulsion between cationic ligands. These findings provide a novel method for tailoring the properties of molecular gold clusters through controlled ligand exchange without requiring the design of thiolate ligands with varying geometrical structures.
- Supakorn Tantisriyanurak, Sorasak Klinyod, Wanwipa Leangsiri, Watinee Nunthakitgoson, Asadawut Soyphet, Marisa Ketkaew, Anawat Thivasasith, Ploychanok Iadrat, Chadatip Rodaum, Thassanant Atithep, Mai Thanh Nguyen, Tetsu Yonezawa, Chularat Wattanakit
“Carbon Nanotubes Deposited on Mordenite Zeoilte/NiAl-Layered Double Hydroxide Composites as Electrocatalysts for 2,5-Furandicarboxylic Acid Production from 5-Hydroxymethylfurfural”
ACS Applied Nano Materials, 6(10), 8784-8794 (2023). 【ACS】(IF = 6.140)
DOI: 10.1021/acsanm.3c01168 (Published (web) 12 May 2023)
【国際共同研究】
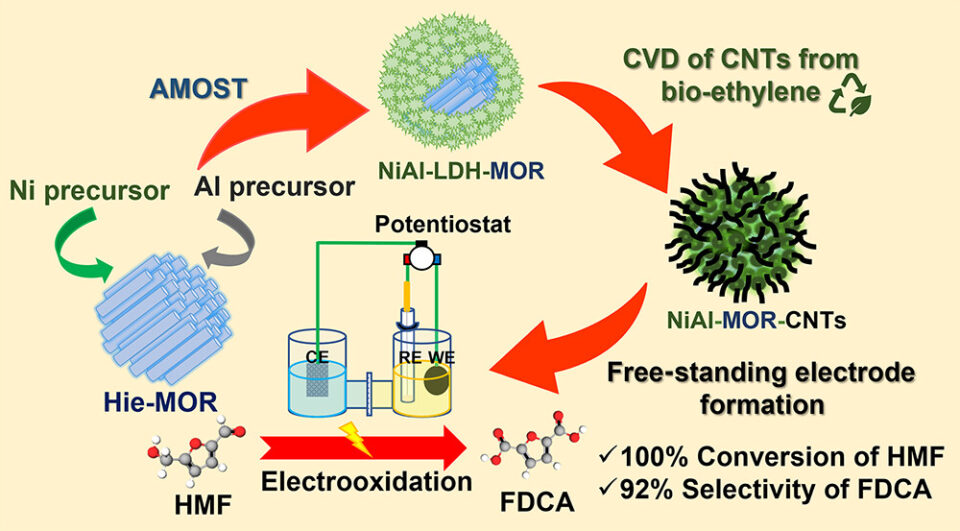
Abstract: The nanostructured free-standing NiAl-MOR-CNTs electrocatalysts containing carbon nanotubes (CNTs) deposited on mordenite (MOR) zeolite/NiAl-layered double hydroxide (NiAl-LDH) have been successfully fabricated. Initially, the core-shell NiAl-LDH-MOR with hierarchical structures was observed via an aqueous miscible organic solvent treatment process to deposit nanosized NiAl-LDH homogeneously on a zeolite surface. The synthesized NiAl-LDH-MOR composites were calcined to produce highly dispersed metal oxides catalyzing the CNTs formation via chemical vapor deposition using bioethanol as a feedstock, eventually producing the NiAl-MOR-CNTs composites. To illustrate the beneficial aspect of the prepared composite, they were mixed with Nafion as a binder and pressed into circular pellets used as free-standing electrodes for electrochemical oxidation of 5-hydroxymethylfurfural (HMF) to 2,5-furandicarboxylic acid (FDCA), an important renewable monomer of bioplastic. The prepared free-standing electrodes exhibited an excellent catalytic performance of 100% conversion of HMF with a high selectivity of FDCA up to 92% under ambient conditions. Moreover, the synthesized electrocatalysts can be recycled even after several consecutive cycles without any loss of catalytic activity, confirming a high stability of the synthesized electrodes. This work illustrates the design of electrocatalysts by using conductive CNTs grown on highly dispersed metal active sites on zeolite/LDH composites without using commercial conductive supports such as nickel foam or carbon fiber paper.
- Wei Jian Sim, Mai Thanh Nguyen, Tetsu Yonezawa
“Noble-Metal Free Zinc-Air Battery Catalysts”【Review】
Materials Transactions, 64(10), 2394-2399 (2023).【JIM】
DOI: 10.2320/matertrans.MT-MH2022008
【研究室内研究】
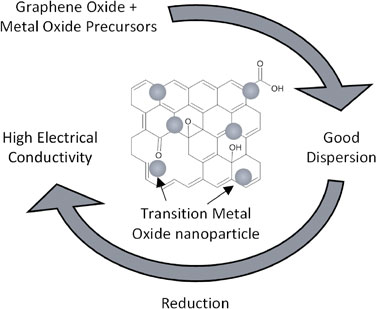
Abstract: Zinc-Air Batteries (ZABs) are a promising solution for grid-scale storage. In this work, ZAB chemistry is reviewed and the role of catalysts at the cathode is explained. Transition metal oxides are an economical substitute for noble-metal based catalysts. However, their poor electrical conductivity requires additional efforts in catalyst activation. This is commonly done by reducing particle size and increasing electrical access which can be done simultaneously by hybridizing with carbon derivatives such as reduced graphene oxide.
- Wathanyu Kao-ian, Jinnawat Sangsawang, Pinit Kidkhunthod, Suttipong Wannapaiboon, Manaswee Suttipong, Amornrat Khampunbut, Prasit Pattananuwat, Mai Thanh Nguyen, Tetsu Yonezawa, Soorathep Kheawhom
“Unveiling the role of water in enhancing the performance of zinc-ion batteries using dimethyl sulfoxide electrolyte and manganese dioxide cathode”
Journal of Materials Chemistry A, 11(20), 10584-10595 (2023). 【RSC】(IF=14.511)
DOI: 10.1039/D3TA01014G (Published (web) 20 April 2023)
【国際共同研究】

Abstract: Due to its excellent reversibility and stability, a dimethyl sulfoxide (DMSO) electrolyte is seen to be a promising nonaqueous electrolyte. Compared to aqueous Zn-MnO2 systems, ZIBs having DMSO electrolytes, and manganese dioxide (MnO2) cathodes display inferior rates and capacity performances. However, it is found that the addition of water can improve the performance of DMSO-based ZIBs, even a small amount of water. In this work, the effect of water on the performance of DMSO-based ZIBs is thoroughly investigated. Herein, the layer-type MnO2 (δ type, birnessite) is used as the main cathode material. Operando X-ray diffraction (XRD) analysis and in-situ X-ray absorption spectroscopy (XAS) results suggest that the existence of water in DMSO electrolytes can lead to changes occurring in the Zn2+ intercalated phase. A Zn-birnessite when replaced by a super-hydrated Zn-buserite provides a much improved solid-phase diffusion of Zn2+ and surface kinetics. The optimized battery yields the highest capacity of 224 mAh/g and can cycle over 2,500 cycles. This discovery extends the understanding of wet nonaqueous electrolyte chemistry and paves the way towards the practical application of ZIBs.
- Phonapha Tangthuam, Wathanyu Kao-ian, Jinnawat Sangsawang, Catleya Rojviriya, Prae Chirawatkul, Jitti Kasemchainan, Falko Mahlendorf, Mai Thanh Nguyen, Tetsu Yonezawa, Soorathep Kheawhom
“Carboxymethyl cellulose as an artificial solid electrolyte interphase for stable zinc-based anodes in aqueous electrolytes”
Materials Science for Energy Technologies, 6, 417-428 (2023). 【Elsevier】【Open Access】(IF=7.63)
DOI: 10.1016/j.mset.2023.04.003 (Published (web) 17 April 2023)
【国際共同研究】
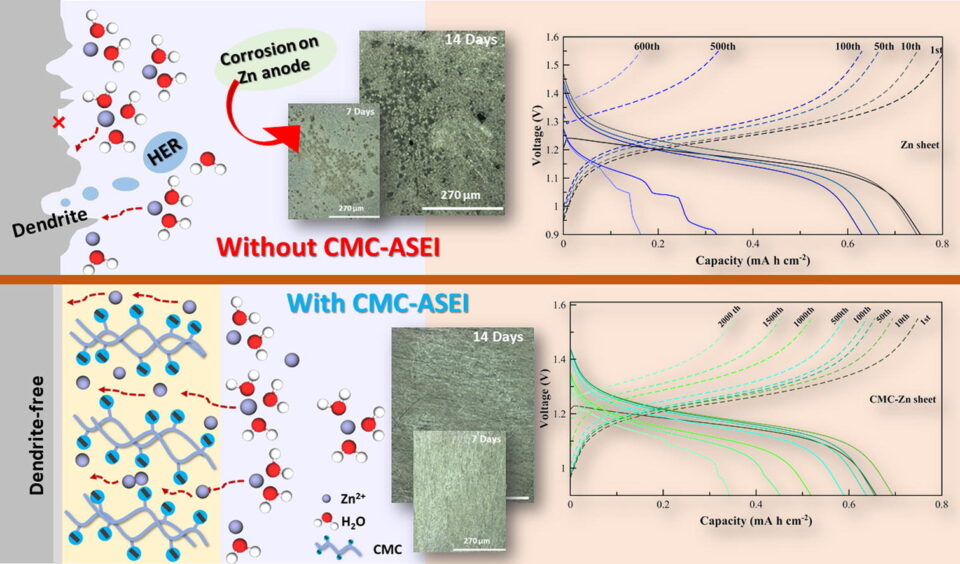
Abstract: Zinc (Zn) is viewed as a promising anode material for large-scale secondary batteries. However, due to parasitic reactions and uneven Zn distribution during repeated stripping/plating cycles, Zn anodes show inferior performance and stability. To overcome such drawbacks, carboxymethyl cellulose (CMC) as an artificial solid electrolyte interphase (ASEI) is fabricated on a Zn sheet and Zn-graphite composite anode. The roles of CMC-ASEI are examined using X-ray tomography, X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS). Results show that the carboxyl group in CMC can regulate the flux and local concentration of Zn ions at the surface, allowing uniform Zn dissolution/deposition, and can suppress corrosion by reducing water activities on the anode’s surface. At 5 mA cm-2, the Zn-iodine battery having CMC-ASEI can cycle up to 2,000 cycles. This work provides a simple and scalable solution for advanced Zn anodes for Zn-based batteries.
- Supattra Somsri, Anittha Prasertsab, Peerapol Pornsetmetakul, Narasiri Maineawklang, Mai Thanh Nguyen, Tetsu Yonezawa, Chularat Wattanakit
“Synthesis of cyclodextrin-stabilized gold nanoparticles supported hierarchical zeolites for the facile production of furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF)”
Microporous and Mesoporous Materials, 354, 112599 (2023).【Elsevier】(IF = 5.876)
DOI: 10.1016/j.micromeso.2023.112559 (Published (web) 24 March 2023)
【国際共同研究】
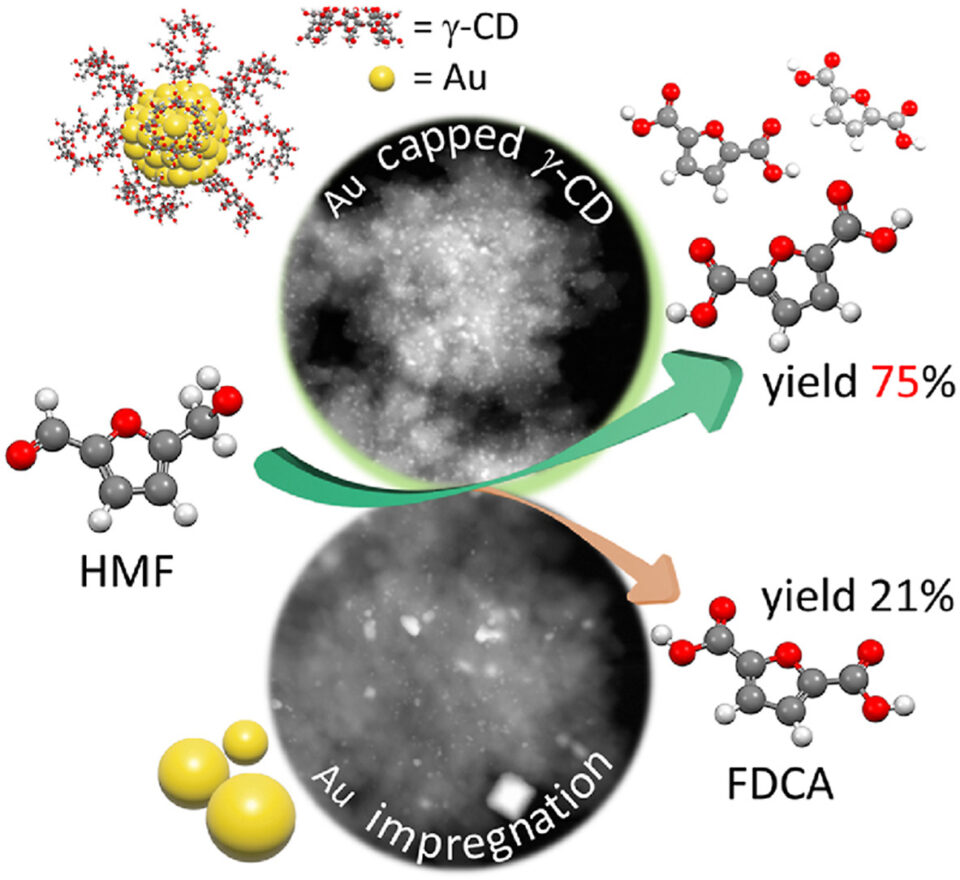
Abstract: The development of gold nanoparticles (Au NPs)-based catalysts is a crucial issue in diverse catalytic applications due to their excellent activity. However, it often suffers from the Au NPs sintering at high temperatures, and therefore the aggregation of Au NPs to large particle sizes results in a decreased active surface area, eventually lowering a catalytic activity. To overcome these limitations, the rational design of small-scale and highly dispersed nanoparticles of gold on oxide supports is a challenging task in heterogeneous catalysis. In this contribution, we report an efficient concept to fabricate ultra-small gold nanoparticles supported on hierarchical ZSM-5 surfaces using cyclodextrin as a stabilizer with the particle size in the range of 1.3 ± 0.4 nm. In strong contrast to this, the large gold particles on zeolite surfaces are obtained approximately 3.6 ± 3.2 nm, when using a conventional impregnation method without a stabilizer. Consequently, the rationally designed ultra-small gold nanoparticles supported on hierarchical ZSM-5 can greatly promote high catalytic performance in HMF oxidation to FDCA with a yield of 75% under mild reaction condition. These findings emphasize the advantage of using cyclodextrin stabilized metallic nanoparticles supported on zeolite surfaces as heterogeneous catalysts for biomonomer production. This example opens up perspectives for the development of highly efficient catalytic materials of gold nanoparticle-zeolite interfaces for biorefinery applications.
- Yuen-ting Rachel Chau, Mai Thanh Nguyen, Tomoharu Tokunaga, and Tetsu Yonezawa
“Mechanistic consideration of ZnTe microspheres formation in a PVP-contained polyol system via hot injection method”
Advanced Powder Technology, 34(4), 103970 (2023). 【Elsevier】(IF=4.833)
DOI: 10.1016/j.apt.2023.103970 (Published (web) 22 February 2023)
【大学間共同研究】
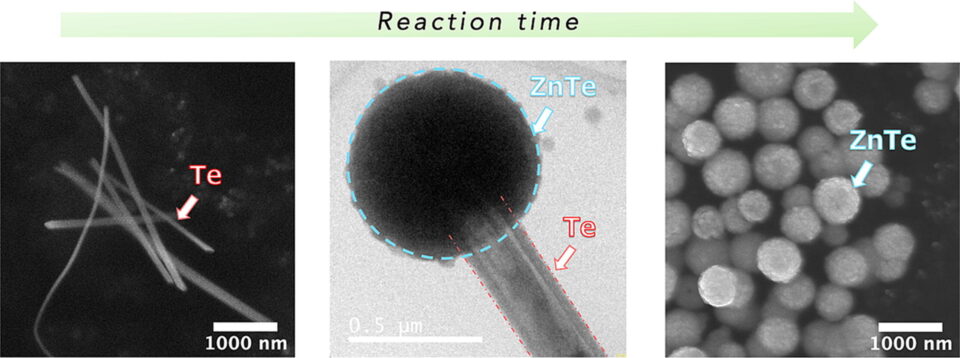
Abstract:Zinc telluride (ZnTe) microspheres have been synthesized in a polyol system with adding various amount of poly(N-vinyl-2-pyrrolidone) (PVP, K-90) at 200 °C via a hot injection method. The smallest sized ZnTe microspheres obtained in this study had an average size of 634 nm through reaction for 48 h using 518 mg of PVP. A retrospective study was conducted to evaluate the formation process of ZnTe microspheres by conducting experiments from 0.2 h to 48 h. Nucleation and growth of ZnTe at the tips of Te rods in a PVP-contained polyol system were observed in sample reacted less than 1 h. More ZnTe microspheres and less Te rods were observed when the reaction was proceeded for longer time, suggesting that Te rods were the sacrificial template for the growth of ZnTe. Amount of PVP is the key factor of controlling dimensions of Te rods formed at the initial stage as well as the sizes of ZnTe microspheres by influencing the nucleation and growth rate of ZnTe. The obtained ZnTe microspheres at 48 h consisted of voids, which were originated by the detachment of ZnTe microspheres from Te rods after their growth.
- Mohan Gopalakrishnan, Sunantha Ganesan, Mai Thanh Nguyen, Tetsu Yonezawa, Supareak Praserthdam, Rojana Pornprasertsuk, Soorathep Kheawhom【Review Article】
“Critical roles of metal-organic frameworks in improving the Zn anode in aqueous zinc-ion batteries”
Chemical Engineering Journal, 457, 141334 (2023).【Elsevier】(IF=16.744)
DOI: 10.1016/j.cej.2023.141334 (Published (web) 4 January 2023)
【国際共同研究】
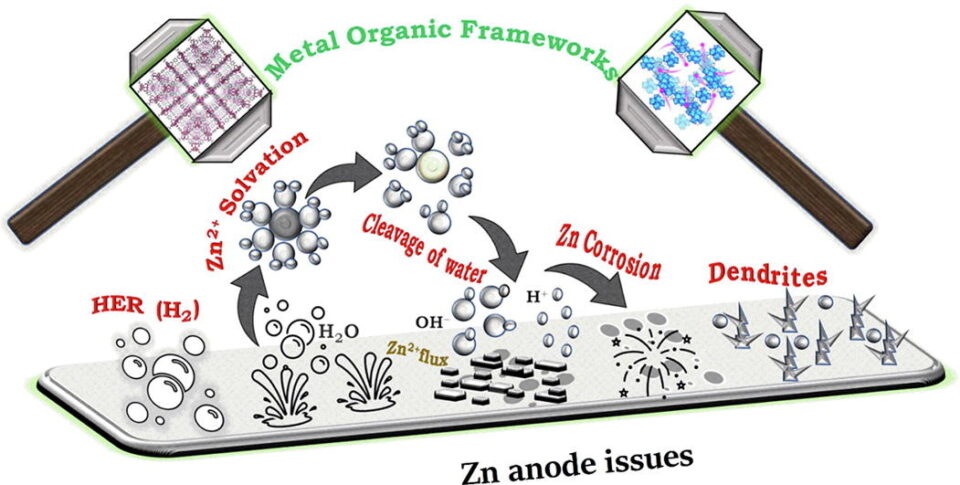
Abstract: Due to their high energy density, safety, and low cost, rechargeable aqueous zinc-ion batteries (AZIBs) have recently gained much interest. Issues, however, such as anode side reactions, passivation, corrosion, hydrogen evolution, and Zn dendrite growth, continue to pose significant barriers to further AZIBs applications. Herein, metal-organic frameworks (MOFs) are presented as potential candidates to suppress the above-mentioned problems effectively. Because of their multifunctional homogeneous porous structure and abundance of active sites with substantial surface areas, MOFs can enhance the performance of the Zn anode materials, electrolytes, and electrolyte additives. First, it emphasizes the inherent chemical characteristics, difficulties, and solvation of Zn anodes. Then, MOFs/MOF-derived anode grids or layers, anode modifications by MOFs and 3D host, MOF-based electrolytes, and separators are classified and compared in terms of structural and electrochemical properties, issues, and solutions. This review aims to provide potential directions and perspectives for the rational design of MOF-based Zn anodes and basic comprehension of the mechanisms affecting Zn2+ solvation in high-performance AZIBs. Finally, the challenges and opportunities of designing MOF-based Zn anodes are proposed to extend the cycling lifetime and promote the commercialization of AZIBs.
- Shiva Rezaei Motlagh, Ramin Khezri, Ahmad Azmin Mohamad, Rojana Pornprasertsuk, Pinit Kidkhunthod, Mai Thanh Nguyen, Tetsu Yonezawa, Soorathep Kheawhom
“Enhancing electrochemical performance and stabilizing zinc anode in mild acidic electrolyte using combined additive”
Materials Science for Energy Technologies, 6, 178-191 (2023).【Elsevier】【Open Access】(IF=7.63)
DOI: 10.1016/j.mset.2022.12.011 (Published (web) 2 January 2023)
【国際共同研究】
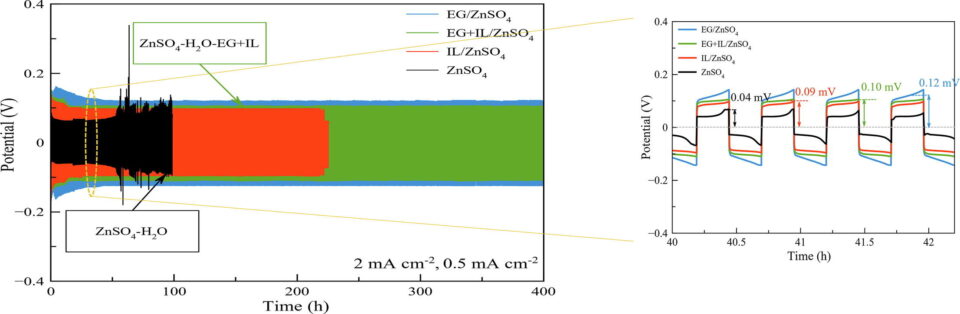
Abstract: Rechargeable zinc-based batteries having aqueous electrolytes are renowned as promising alternatives for large scale energy storage systems (ESSs). However, in mild acidic electrolytes, zinc anodes pose several issues that have yet to be overcome. For tackling the major difficulties of zinc anodes, electrolyte engineering is the most practical and cost-effective strategy. Notwithstanding the broad variety of mechanisms associated with different electrolyte additives, the function of a single additive is straightforward. Finding combined additives with broader functionalities and greater efficacy is crucial. In this work, the combination of ionic liquid (IL) 1-butyl-1-methylpyrrolidinium dicyanamide ([BMPY][DCN]) and ethylene glycol (EG) as additives in an aqueous electrolyte 3 M ZnSO4 proves to be an effective strategy for simultaneously enhancing both zinc dissolution and deposition. IL significantly enhances zinc (Zn) dissolution and deposition while EG plays an important role in suppressing hydrogen evolution reaction (HER)and corrosion. Thus, 13.50% improvement in capacity is achieved by the mixed additive EG+IL/ZnSO4 compared with the 3 M ZnSO4 electrolyte. In the Zn//Zn symmetrical system at 2 mA cm-2, the mixed additive greatly augments the cyclability of zinc, reaching over 400 h while maintaining excellent coulombic efficiency (CE). In-situ XAS and FT-EXAFS analyses confirm the high activities of both zinc oxidation/reduction in the mixed additive electrolyte. Results demonstrate that EG+IL/ZnSO4 can be applied to increase the cyclability of aqueous zinc-based batteries while sustaining high performance.
書籍
- 微粒子分散・凝集講座 第1巻
「分散凝集の基礎」
一般社団法人 日本ディスパージョンセンター 監修
編者:米澤 徹、武田真一、藤井秀司、石田尚之
近代科学社Digital
ISBN978-4-7649-6061-9
(2023.8.25 初版発行)
