Our staffs: Names in Blue, Our students: Names in Red.
Original Papers
- Lyn Marie De Juan-Corpuz, Ryan Dula Corpuz, Anongnat Somwangthanaroj, Mai Thanh Nguyen, Tetsu Yonezawa, Jianmin Ma, Soorathep Kheawhom
“Binder-free centimeter-long V2O5 nanofibers on carbon cloth as cathode material for zinc-ion batteries”
Energies, 13(1), 31 (2020). 【Open Access】
DOI: 10.3390/en13010031 (Published 19 December 2019)
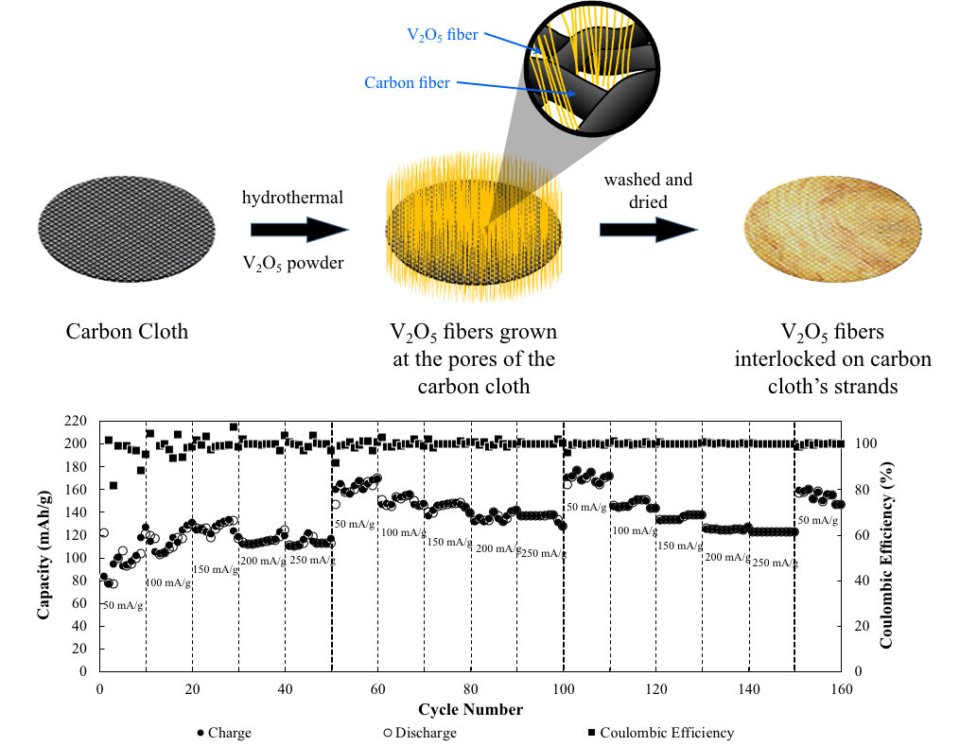
Abstract: Recently, rechargeable aqueous zinc-ion batteries (AZBs) have attracted extensive interest due to their safety, abundance, low cost, and low toxicity. However, aqueous electrolytes require a polymeric binder to prevent dissolution of the active material in addition to its binding properties. This study highlights binder-free, centimeter long, single-crystal, V2O5 nanofibers (BCS-VONF) on carbon cloth, as the cathode material for AZBs synthesized via a simple one-step hydrothermal process. BCS-VONF in 3.0 M Zn(OTf)2 exhibit promising electrochemical performance with excellent capacity retention. Even in the absence of a binder, BCS-VONF were found to be very stable in 3.0 M Zn(OTf)2. They will not yield to the dissolution and detachment of the active material on the current collector. The novel strategy described in this study is an essential step for the development of BCS-VONF on carbon cloth, as a promising cathode material for AZBs.
- Ryan Dula Corpuz, Lyn Marie Z. De Juan, Supareak Praserthdam, Rojana Pornprasertsuk, Tetsu Yonezawa, Mai Thanh Nguyen and Soorathep Kheawhom
“Annealing induced a well-ordered single crystal δ-MnO2 and its electrochemical performance in zinc-ion battery”
Scientific Reports, 9, 15107 (2019).
DOI: 10.1038/s41598-019-51692-x (Published 22 October 2019)Abstract: Herein, the formation and electrochemical performance of a novel binder-free turbostratic stacked/ well-ordered stacked δ-MnO2-carbon fiber composite cathodes in deep eutectic solvent (DES) based zinc-ion battery (ZIB) is reported. Results of morphological, elemental, and structural analyses revealed directly grown and interconnected δ-MnO2 crumpled nanosheets on a carbon fiber substrate. Moreover, an improvement via a simple annealing strategy in the stacking, surface area and conductivity of the δ-MnO2 sheets was observed. Annealing induces the rearrangement of δ-MnO2 sheets resulting in the transformation from turbostratic stacking to a well-ordered stacking of δ-MnO2 sheets, as indicated by the selected area electron diffraction (SAED) hexagonal single crystal pattern. Besides, the formation of the well-ordered stacking of δ-MnO2 sheets exhibited improved electrochemical performance and cyclability, as cathode material for ZIB. The novel strategy described in this study is an essential step for the development of binder-free δ-MnO2-C fiber composite with a well-ordered stacking of δ-MnO2 sheets. This study also demonstrated comparable electrochemical performance between the turbostratic δ-MnO2 sheets and the well-ordered stacked δ-MnO2 sheets.
- Soraya Hosseini, Ali Abbasi, Luc-Olivier Uginet, Nicolas Haustraete, Supareak Praserthdam, Tetsu Yonezawa, and Soorathep Kheawhom
“The Influence of Dimethyl Sulfoxide as Electrolyte Additive on Anodic Dissolution of Alkaline Zinc-Air Flow Battery”
Scientific Reports, 9, 14958 (2019).
DOI: 10.1038/s41598-019-51412-5 (Published 18 October 2019)Abstract: The present work describes the effects of dimethyl sulfoxide (DMSO) in KOH aqueous electrolyte on the performance of a zinc-air flow battery. Aqueous electrolytes containing 7 M KOH and (0 to 20)% v/v DMSO were studied revealing a critical role of DMSO on the dissolution and deposition of zinc. The anodic zinc dissolution process was studied via cyclic voltammetry, Tafel polarization and electrochemical impedance spectroscopy (EIS). The presence of DMSO showed improved zinc dissolution performance with the highest peak of zinc dissolution being the electrolyte containing 5% v/v DMSO. Tafel analysis demonstrated a significant decrease in polarization resistance and an increase in corrosion rate due to the introduction of DMSO to the electrolyte. This suggests that DMSO has the ability to suspend zinc oxide in the electrolyte, thus preventing passivation of the zinc surface. EIS results revealed that by adding DMSO to the electrolyte, charge transfer resistance increased. This is attributed to the formation of passive layers having arisen from DMSO adsorption, the formation of zincate ions in the vicinity of the zinc surface, and the deposition of discharged products. A difference in Nyquist plots was observed for 20% v/v DMSO/KOH and 0% v/v DMSO/KOH electrolytes implying non-Debye relaxation behavior taking place due to the surface effects. The electrolytes were implemented in a zinc-air flow battery. Maximum power densities of 130 mW/cm2 (5% v/v DMSO) and 125 mW/cm2 (20% v/v DMSO) were obtained and were observed to be about 43% and 28% higher than that of the DMSO-free electrolyte. Results indicated that when 20% v/v DMSO was added to KOH solution, there was 67% zinc utilization efficiency (550 mAh/g) which provided 20% improvement in discharge capacity. Further, the battery with 20% v/v DMSO demonstrated excellent cyclability. Overall, DMSO shows great promise for enhancement of zinc dissolution/deposition in zinc-air batteries.
- Mai Thanh Nguyen, Kai Yu, Tomoharu Tokunaga, Kornkit Boonyaperm, Soorathep Kheawhom, Masashi Arita, and Tetsu Yonezawa
“A Green Synthesis of Size-Tunable Iron Oxides and Iron Nanoparticles in Salt Matrix”
ACS Sustainable Chemistry and Engineering, 7(21), 17697-17705 (2019).
DOI: 10.1021/acssuschemeng.9b03950 (Published (web) 4 October 2019)Abstract: This research is devoted for the synthesis of Fe oxide and Fe nanoparticles (NPs) via respectively thermal decomposition of iron(III) oleate and H2 reduction of either iron(III) oleate or iron oxide nanoparticles in NaCl matrix. Lowering the weight ratios of metal precursor to NaCl and synthesis temperature results in smaller particle sizes. A higher uniformity of Fe NPs can be achieved with a two-step synthesis, this is, thermal decomposition of iron oleate followed by H2 reduction of iron oxides, with the milling of iron oxides and NaCl prior to the reduction step. Fe NPs obtained in this study are stable in the air for long time storage. It is found that thin oxide and C layers on particle surface are responsible for their stability.
- Kunihiro Narita, Yohei Ishida, Tetsu Yonezawa, and Zhong Huang
“Super Polycationic Molecular Compounds: Au144(SR+)60 Clusters”
The Journal of Physical Chemistry C, 123(35), 21768-21773 (2019).
DOI: 10.1021/acs.jpcc.9b05316 (Published (web) 13 Aug 2019)

Abstract: We have recently pioneered per-cationized molecular gold (Au) cluster compounds. Herein, we present a new series of per-cationized Au cluster compounds, Au144(SR+)60, which is the largest reported compound with the most stable and studied Au clusters. Although it is typically difficult to obtain a single composition of an Au144 cluster due to the existence of similar-sized quasi-stable compounds, optimized thermal etching and selective precipitation made strict size focusing into a single Au144 composition possible and produced Au144(SR+)60 with high atomic precision. In positive-mode high-resolution ESI-MS, per-cationized Au144(SR+)60 clusters with different numbers of PF6− counter anions for the +12 to +21-charged states were observed. The Au144(SR+)60, 60(+) termini distributed over a spherical surface of radius ~ 2.5 nm, presented here is, to the best of our knowledge, the most polycationic molecular compound reported thus far.
- Wan-Yu Chung, Yi-Chin Lai, Tetsu Yonezawa,* and Ying-Chih Liao*
“Sintering Copper nanoparticles with photonic additive for printed conductive patterns by intense pulsed light”
Nanomaterials, 9(8), 1071 (2019).
DOI: 10.3390/nano9081071 (Published 25 July 2019)Abstract: In this study, an ink formulation was developed to prepare conductive copper thin films with compact structure by using intense pulsed light (IPL) sintering. To improve inter-particle connections in the sintering process, a cuprous oxide shell was synthesized over copper nanoparticles (CuNP). This cuprous oxide shell can be reduced by IPL with the presence of a reductant and fused to form connection between large copper particles. However, the thermal yield stress after strong IPL sintering resulted in cracks of conductive copper film. Thus, a multiple pulse sintering with an off time of 2 s was needed to reach a low resistivity of 10−5 Ω·cm. To increase the light absorption efficiency and to further decrease voids between CuNPs in the copper film, cupric oxide nanoparticles (CuONP) of 50 nm, were also added into ink. The results showed that these CuONPs can be reduced to copper with a single pulse IPL and fused with the surrounding CuNPs. With an optimal CuNP/CuONP weight ratio of 1/80, the copper film showed a lowest resistivity of 7 × 10−5 Ω·cm, ~25% conductivity of bulk copper, with a single sintering energy at 3.08 J/cm2. The ink can be printed on flexible substrates as conductive tracks and the resistance remained nearly the same after 10,000 bending cycles.
- Min JIa Saw, Batu Ghosh, Mai Thanh Nguyen, Kridsada Jirasattayaporn, Soorathep Kheawhom, Naoto Shirahata,* and Tetsu Yonezawa*
“High Aspect Ratio and Post-processing Free Silver Nanowires as Top Electrodes for Inverted-structured Photodiode”
ACS Omega, 4(8), 13303-13308 (2019).
DOI: 10.1021/acsomega.9b01479 (Published (web) 5 Aug 2019)

Abstract: Silver nanowires (Ag NWs) as transparent conducting electrodes are widely used in many applications such as organic light-emitting diodes (OLEDs), polymer light-emitting diodes, touch screens, solar cells, and transparent heaters. In this work, using a large-scale synthesis, the synthesized Ag NWs had a high aspect ratio of 2820. The Ag NWs could be applied as a top transparent electrode in a device by simple drop-casting without any post-processing steps. The fabricated device comprised 4,4′-bis(carbazol-9-yl)biphenyl/MoO3 organic/inorganic layers which are parts of the inverted structure OLEDs or solar cells. The photodiode characteristics at the UV range were observed in the device. The ability of Ag NWs to replace opaque metals as top electrodes in a device has been demonstrated.
- Zhong Huang, Yohei Ishida,* and Tetsu Yonezawa*
”Basic [Au25(SCH2CH2Py)18]−·Na+ Clusters: Synthesis, Layered Crystallographic Arrangement, and Unique Surface Protonation”
Angewante Chemie International Edition, 55(38), 13411-13415 (2019).
DOI: 10.1002/anie.201908905 10.1002/ange.201908905 (Published (web) 19 July 2019)Abstract: We report the first synthesis of high‐purity and high‐yield Au₂₅ clusters protected by the basic pyridyl ethanethiol (HSCH2CH2Py, 4-PyET and 2-PyET). Single-crystal X-ray diffraction of the [Au25(4-PyET)18]−·Na+ clusters has revealed a structure similar to that known for the phenyl ethanethiolate analog, but with pyridyl-N coordination to Na+, a more relaxed ligand shell, and a profoundly layered arrangement in the solid state. Because of the pendant Py moiety, the [Au25(4-PyET)18]− clusters are endowed with a unique (de)protonation equilibria, which has been characterized in detail by UV-vis absorption and 1H-NMR spectroscopy.
[Au25(4-PyET)18]− clusters showed an unexpectedly H+-dependent solubility that is tunable in aqueous and organic solvents. The successful synthesis of the basic Py-terminated thiolate-protected Au25 clusters paves the way to realize a new family of metalloid clusters possessing basic properties. - Min Jia Saw, Mai Thanh Nguyen, Shilei Zhu, Yongming Wang, and Tetsu Yonezawa
“Synthesis of Sn/Ag-Sn nanoparticles via room temperature galvanic reaction and diffusion”
RSC Advances, 9, 21786-21792 (2019).
DOI: 10.1039/C9RA02987G (Published on 12 July 2019)
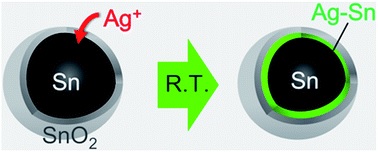
Abstract: Tin (Sn) has a low melting temperature, i.e., 231.9 °C for the bulk, and the capability to form compounds with many metals. The galvanic reaction between Sn nanoparticles (NPs) as the core and silver nitrate at room temperature under argon gas in an organic solvent without any reducing power, was employed for the first time to coat an Ag–Sn intermetallic shell, i.e., Ag3Sn and/or Ag4Sn, on Sn NPs. For spherical Sn NPs, the NPs retained a spherical shape after coating. Uniform and Janus structures consisting of a β-Sn core with Ag–Sn shell were observed in the resulting NPs and their population related to the input molar ratios of the metal precursors. The observation of the intermetallic shell is general for both spherical and rod-shape Sn NPs. The formation of the intermetallic shell indicated that two reactions occurred sequentially, first reduction of Ag ions to Ag atoms by the Sn core, followed by interdiffusion of Ag and Sn to form the Ag–Sn intermetallic shell.
- Lyn Marie Z. De Juan-Corpuz, Mai Thanh Nguyen, Ryan D. Corpuz, Tetsu Yonezawa,* Nataly Carolina Rosero-Navarro, Kiyoharu Tadanaga, Tomoharu Tokunaga, and Soorathep Kheawhom
“Porous ZnV2O4 Nanowire for Stable and High-rate Lithium Ion Battery Anodes”
ACS Applied Nano Materials, 2(7), 4247-4256 (2019).
DOI: 10.1021/acsanm.9b00703 (Published (web) 14 June 2019)
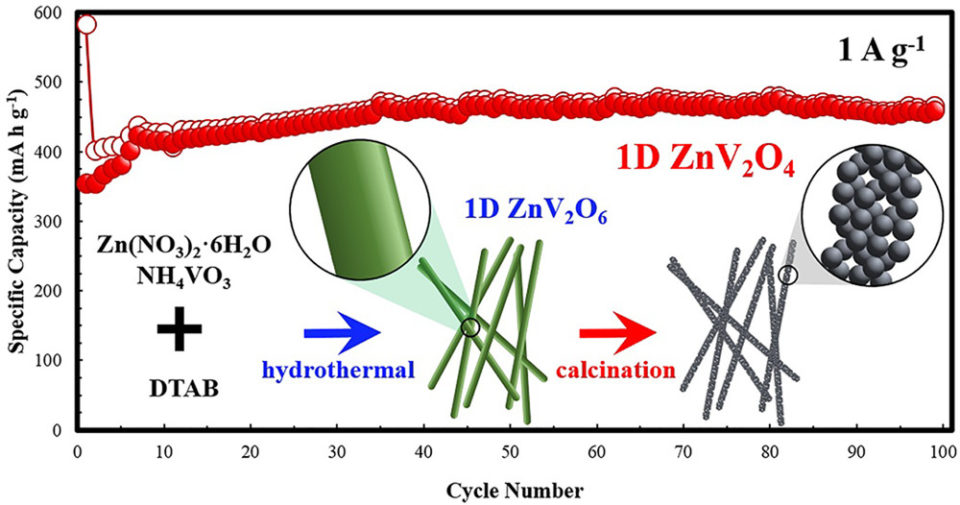
Abstract: Porous ZnV2O4 nanowires (NWs) were successfully prepared by hydrothermal reaction followed by calcination. Despite the porous structure, these porous ZnV2O4 NWs are single crystal with {220} facets and wire direction along <111>. Based on the electrochemical test, these porous ZnV2O4 NWs have better cycling stability and higher specific capacity, i.e., 460 mA h g-1 after 100 cycles and 149 mA h g-1 after 1000 cycles using 1 and 5 A g-1 current densities respectively, compared to other morphologies, i.e., spherical and coral-like morphologies. As ternary transition metal oxide, the produced porous ZnV2O4 NWs undergoes phase transformation without compromising the resulting capacity. On the other hand, the CV curves at different scan rates indicate a pseudo-capacitive electrochemical behavior of the porous ZnV2O4.
- Rasu Muruganantham, Irish Valerie Maggay, Lyn-Marie Z. De Juan, Tetsu Yonezawa, Mai Thanh Nguyen, Yan-Gu Lin, Chia-Her Lin, and Wei-Ren Liu
“Electrochemical Exploration of Calcination Temperature Effects of Mesoporous Zinc Vanadate Anode Material for Na-Ion Battery”
Inorganic Chemistry Frontiers, 6(10), 2653-2659 (2019).
DOI: 10.1039/C9QI00494G (Published (web) 18 June 2019)

Abstract: Nowadays, transition metal oxide is rapidly developed for Na-ion storage anode materials, which provides relatively high theoretical capacity than the graphitic anode. However, the evaluations of enhanced electrochemical performance of NIBs are ongoing progress via various approaches such as coating or doping and so on. Hence this work, Spinel structure bimetal oxide ZnV2O4 mesoporous material is successfully synthesized via solvothermal technique followed by calcined at different temperatures. The impacts of calcination temperatures on the Na-ion storage anode performance are thoroughly investigated for the first time. The initial discharge capacities of 178, 251, 296 mAh∙g-1 are obtained for 500, 600, and 700°C, respectively. After 250 cycles, ZVO-700 electrode exhibits to retain 166 mAh.g-1 at 200 mA∙g-1 with a high coulombic efficiency of 99%. Meanwhile, ZVO-500 and ZVO-600 retained 55 mAh∙g-1 and 99 mAh∙g-1 with ~27% and ~42% retention rate, respectively. The electrochemical Na-ion storage is predicted by the conversion reaction of ZnV2O4. Moreover, the ZVO-700 sample showed a higher surface area and pore volume than that of ZVO-500 and ZVO-600 °C samples, which leads to remarkable electrochemical performance.
- Sonti Khamsanga, Rojana Pornprasertsuk, Tetsu Yonezawa, Ahmad Azmin Mohamad and Soorathep Kheawhom
”δ-MnO2 nanoflower/graphite cathode for rechargeable aqueous zinc ion batteries”
Scientific Reports, 9, 8441 (2019).
DOI: 10.1038/s41598-019-44915-8 (Published 11 June 2019)Abstract: Manganese oxide (MnO2) is one of the most promising intercalation cathode materials for zinc ion batteries (ZIBs). Specifically, a layered type delta manganese dioxide (δ-MnO2) allows reversible insertion/extraction of Zn2+ ions and exhibits high storage capacity of Zn2+ ions. However, a poor conductivity of δ-MnO2, as well as other crystallographic forms, limits its potential applications. This study focuses on δ-MnO2 with nanoflower structure supported on graphite flake, namely MNG, for use as an intercalation host material of rechargeable aqueous ZIBs. Pristine δ-MnO2 nanoflowers and MNG were synthesized and examined using X-ray diffraction, electron spectroscopy, and electrochemical techniques. Also, performances of the batteries with the pristine δ-MnO2 nanoflowers and MNG cathodes were studied in CR2032 coin cells. MNG exhibits a fast insertion/extraction of Zn2+ ions with diffusion scheme and pseudocapacitive behavior. The battery using MNG cathode exhibited a high initial discharge capacity of 235 mAh/g at 200 mA/g specific current density compared to 130 mAh/g which is displayed by the pristine δ-MnO2 cathode at the same specific current density. MNG demonstrated superior electrical conductivity compared to the pristine δ-MnO2. The results obtained pave the way for improving the electrical conductivity of MnO2 by using graphite flake support. The graphite flake support significantly improved performances of ZIBs and made them attractive for use in a wide variety of energy applications.
- Shilei Zhu, Mai Thanh Nguyen, Tomoharu Tokunaga, and Tetsu Yonezawa
“Size-tunable Alumina-encapsulated Sn-based Phase Change Materials for Thermal Energy Storage”
ACS Applied Nano Materials, 2(6), 3752-3760 (2019).
DOI: 10.1021/acsanm.9b00649 (Published (web) 3 June 2019)
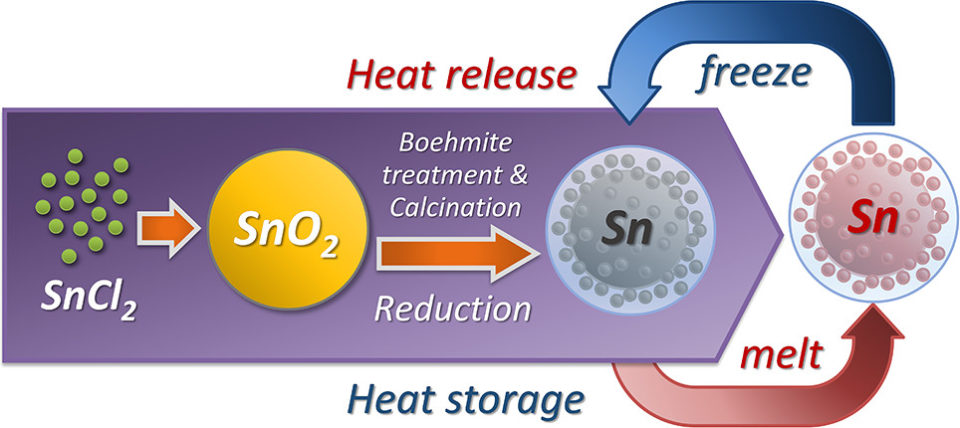
Abstract: The most commonly used phase change materials (PCMs), like organic compounds and inorganic salts, were limited in application by their low thermal conductivity. Herein, for the first time, alumina-encapsulated metallic Sn-based PCMs, named Sn@Al2O3, were successfully fabricated with tunable size (60–2000 nm) by a facile process from low-cost chemicals. The robust fabrication process consists of a surfactant-free solvothermal synthesis of SnO2 spheres, boehmite treatment on SnO2 spheres, calcination in the air, and the final hydrogen reduction to transform SnO2 to metallic Sn, endowing the PCMs with high potential for mass production. The as-obtained Sn@Al2O3 showed a core–shell structure, in which a main metallic Sn core located in the center covered with an Al2O3 shell with small Sn nanoparticles distributed inside. The boehmite treatment, in which the penetration of aluminum species into SnO2 spheres played an important role, was found to be responsible for the unique structure formation of final Sn@Al2O3. The understanding of structure formation mechanism gives the possibilities of a new facile way for the synthesis of metal nanoparticles and particle-distributed nanostructures. The obtained Sn@Al2O3 particles not only have high PCM content (92.37 wt %) but also show a stable thermal behavior and morphology during 100 melt–freeze cycles in the air atmosphere. Furthermore, the low melting temperature of the PCM core, combined with high thermal conductivity of both core material (Sn, 66.8 W m–1 K–1) and shell material (Al2O3, 35 W m–1 K–1), makes Sn@Al2O3 potentially suitable for rapid thermal energy storage in the range 100–300 °C.
- Lianlian Deng, Mai Thanh Nguyen, Shuang Mei, Tomoharu Tokunaga, Masaki Kudo, Syo Matsumura, and Tetsu Yonezawa
“Preparation and growth mechanism of Pt/Cu alloy nanoparticles by sputter deposition onto a liquid polymer”
Langmuir, 35(25), 8418-8427 (2019).
DOI: 10.1021/acs.langmuir.9b01112 (Published (web) 1 June 2019)
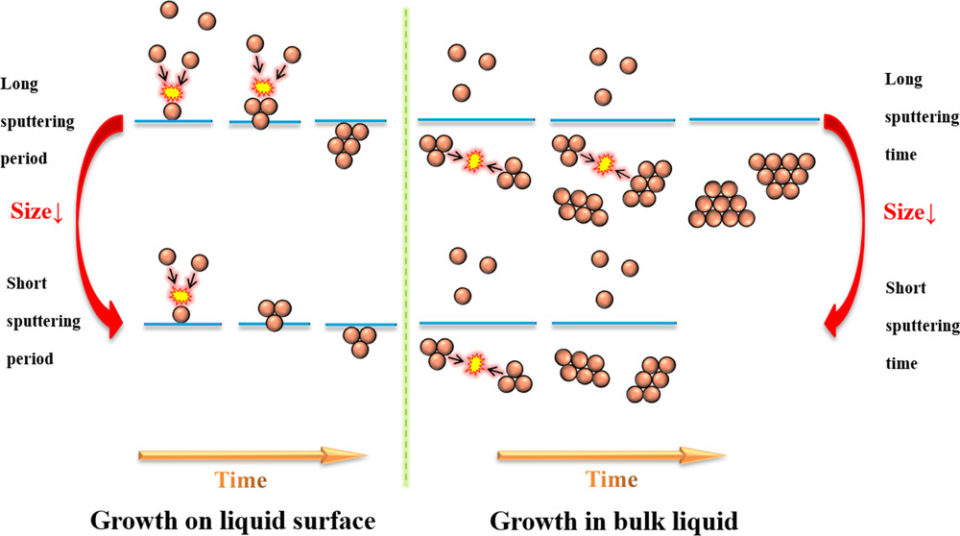
Abstract: We use a green sputtering technique to deposit a Pt/Cu alloy target on liquid polyethylene glycol, PEG, to obtain well-dispersed and stable Pt29Cu71 alloy nanoparticles (NPs). The effects of sputtering current, rotation speed of the stirrer, sputtering time, sputtering period, and temperature of PEG on the particle size are studied systematically. Our key results demonstrate that the aggregation and growth of Pt/Cu alloy NPs occurred at the surface as well as inside the liquid polymer after the particles landed on the liquid surface. According to particle size analysis, a low sputtering current, high rotation speed for the stirrer, short sputtering period, and short sputtering time are found to be favorable for producing small-sized single crystalline alloy NPs. On the other hand, varying the temperature of the liquid PEG does not have any significant impact on the particle size. Thus, our findings shed light on controlling NP growth using the newly developed green sputtering deposition technique.
- Yuan-Ting Rachel Chau, Lianlian Deng, Mai Thanh Nguyen, and Tetsu Yonezawa
“Monitor the Growth and Oxidation of Cu-nanoparticles in PEG after Sputtering”
MRS Advances, 4(5-6), 305-309 (2019).
DOI: 10.1557/adv.2019.55 (Published (web) 24 January 2019)Abstract: Metallic Copper nanoparticles (Cu NPs) were obtained via sputtering of Cu target onto liquid polymer, i.e., poly(ethylene glycol), PEG, under vacuum condition. The Cu NPs growth significantly right after the sputtering deposition from 3.1 nm to 4.1 nm in 4 hours as monitored by TEM. There was negligible growth of NPs for longer time and completely PEG acts as the coating material of Cu NPs so no agglomeration was observed for 1 week. The challenge of characterization of Cu NPs was also discussed.
- Tetsu Yonezawa, Jiajia Shi, Hiroki Tsukamoto, and Mai Thanh Nguyen
“Size-controlled Preparation of Alkylamine-stabilized Copper Fine Particles from Cupric Oxide (CuO) Micro-particles”
MRS Advances, 4(7), 413-418 (2019).
DOI: 10.1557/adv.2019.91 (Published (web) 1 February 2019)Abstract: Size control of copper fine particles is highly important for their application for conductive materials. In this study, easy size tuning of the copper fine particles coated by n-hexylamine was achieved via controlling the ratio of n-hexylamine and the precursor CuO. The obtained particles were stable and had a hydrophobic surface. TG-DTA measurement revealed the formation of thin layer of n-hexylamine on the particles.
Review Papers in Japanese
- 米澤 徹
「低温焼結接合に向けた銅材料の開発」
車載テクノロジー、7(3), pp (2019). - 米澤 徹
「金属微粒子・ナノ粒子・クラスター ~実用研究・基礎研究・楽しみ方~」
C & I Commun, 44(3), 27-32 (2019). - 米澤 徹・松原正樹
「銅微粒子の分散と導電性インク・ペーストへの展開」
色材協会誌、印刷中
“Dispersion of Copper Fine Particles for Conductive Inks and Pastes”
Journal of the Japan Society of Colour Material, in press.
