The professor Yonezawa began his research on gold nanoparticles and noble metal nanoparticles when he was still a student. He has proven that it is possible to create nanoparticles with various potentials by controlling the particle size, structure, and atomic distribution. It is now becoming possible to “design” nanoparticles.
Structure control and behaviors of noble metal nanoparticles and bimetallic nanoparticles
Our lab has a long history of synthesizing nanoparticles that contain palladium, platinum, and gold. When Professor Yonezawa began his research on the subject, the word “nanoparticle” did not even exist. As nanoparticles were often discussed at chemical engineering conferences (and still are), we focused on the “liquid reduction process” and obtained nanoparticles as a dispersion liquid.
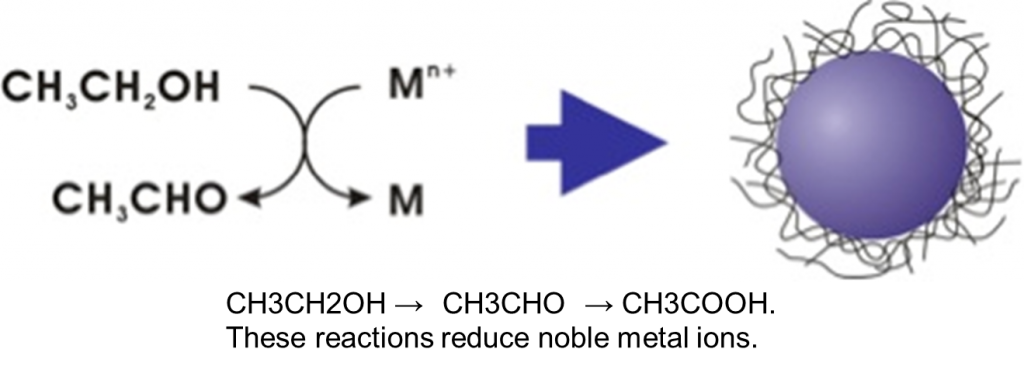
Among them, we have paid particular attention to polyvinyl pyrrolidone (PVP)-protected noble metal nanoparticles and bimetallic nanoparticles and studied them in detail. We used the alcohol reduction process to synthesize the noble metal nanoparticles. Alcohol reduction is a type of chemical reduction process in which a noble metal salt is resolved in an ethanol-water mixture solvent and then heated to reflux. In the process, alcohol is oxidized to aldehyde and then to carboxylic acid, as illustrated below. This is the process that metabolizes alcohol when you drink! As you can see, it is known that α-hydrogen is involved in the reduction process.
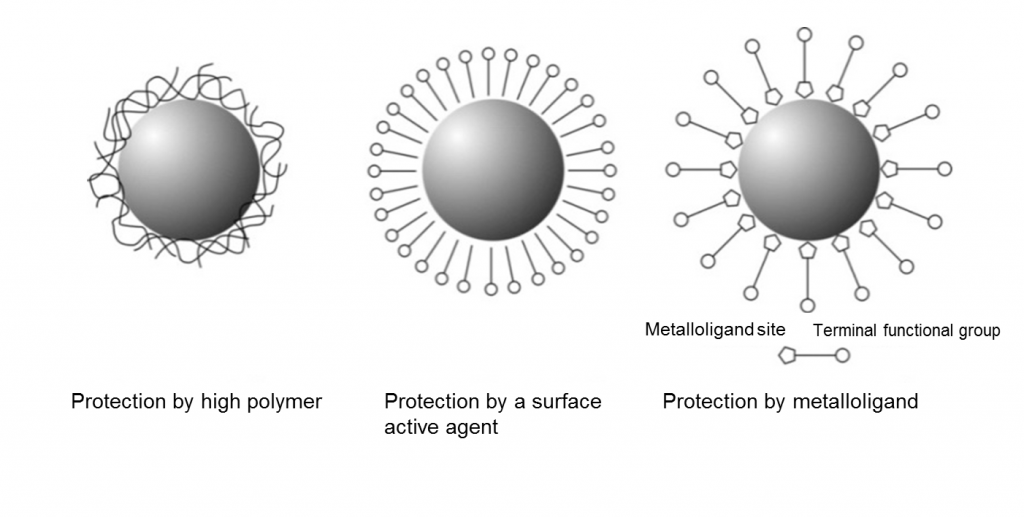
Palladium chloride, chloroplatinic acid, chloroauric acid, silver nitrate, and rhodium chloride are often used as the noble metal salt. By resolving PVP as a stabilizing polymer, we can prepare a dispersion liquid of nanoparticles whose surface is protected by polyvinyl pyrrolidone (PVP). Each nanoparticle is thought to be protected as shown in the drawing on the left. Because the PVP is in contact with the nanoparticle at multiple points, the nanoparticle will not be destabilized unless the PVP is removed from all points simultaneously. Thus, polymer-stabilized nanoparticles are stable.
The alcohol reduction process is a simple method that can be applied to many types of noble metal nanoparticles and can be considered a next-generation method of alcohol reduction. The high-polymer noble metal nanoparticles obtained in these ways function as effective catalysts.
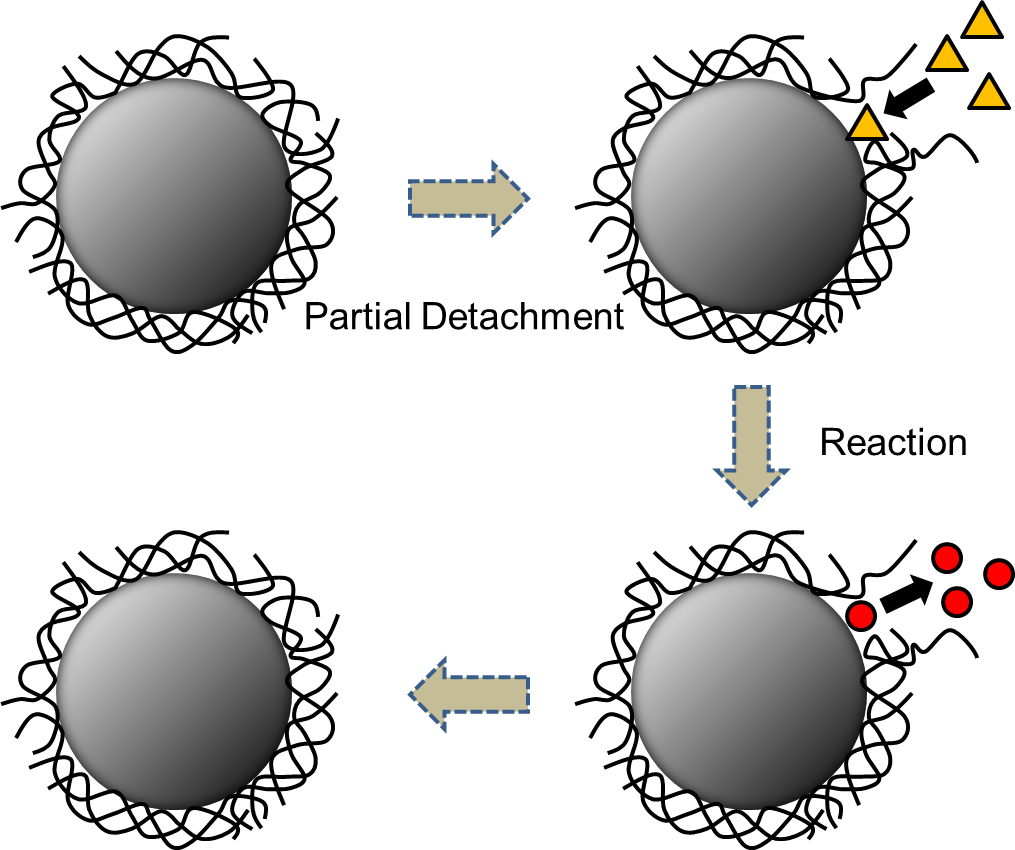
Unlike the nanoparticles protected by the metal metallolig and on the right side of the diagram, nanoparticles protected by a high polymer have a lower force of interaction between the metal surface and high polymer. Therefore, when a substrate (a substance that reacts with a catalyst) approaches the surface of the nanoparticle, the high polymers will be detached from the surface, and when a reaction product moves away, the polymer again begins protecting the nanoparticle. This process repeats, dispersing nanoparticles in a stable manner.
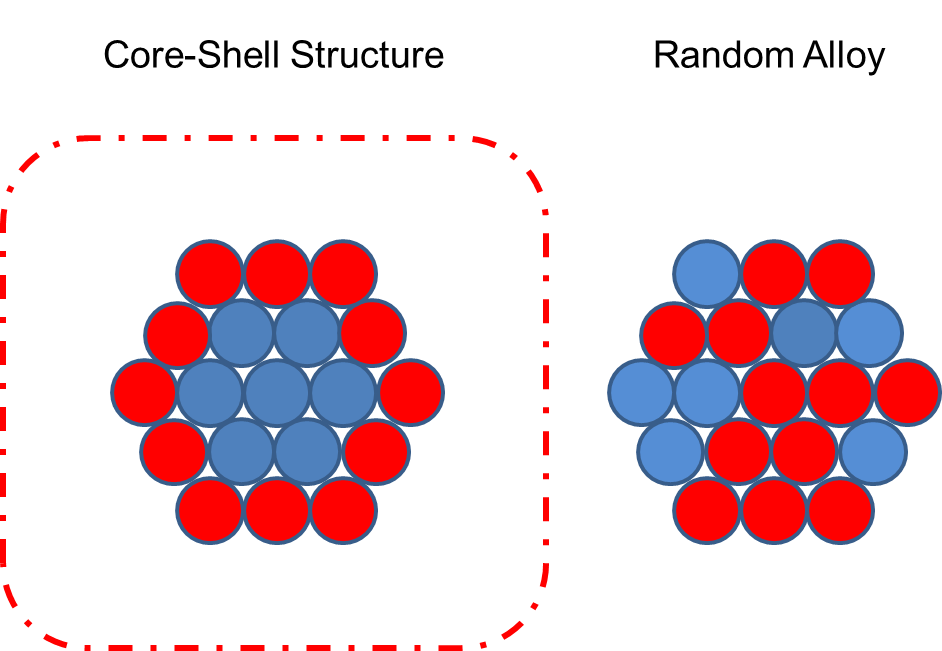
What happens if two metal salts are reduced simultaneously? Just like in a solid solution, one may expect two metal atoms to be disposed randomly; however, this was not the case in reality. In our research, we examined the simultaneous reductions of palladium and platinum. We used X-Ray diffraction (XRD), X-Ray photoemission spectroscopy (XPS), and extended X-ray absorption fine structure (EXAFS) spectroscopy for structural characterization. The XRD patterns of the alloy of these two metals significantly differed from the XRD patterns of the palladium or platinum nanoparticles. This result strongly indicates that a nanoparticle of the alloy contains the two metal atoms.
The simultaneous reductions of palladium and platinum in the ethanol-water mixture in the presence of PVP produce bimetallic nanoparticles of the two metals. We obtained bimetallic nanoparticles of palladium and gold and those of gold and platinum in the same manner.
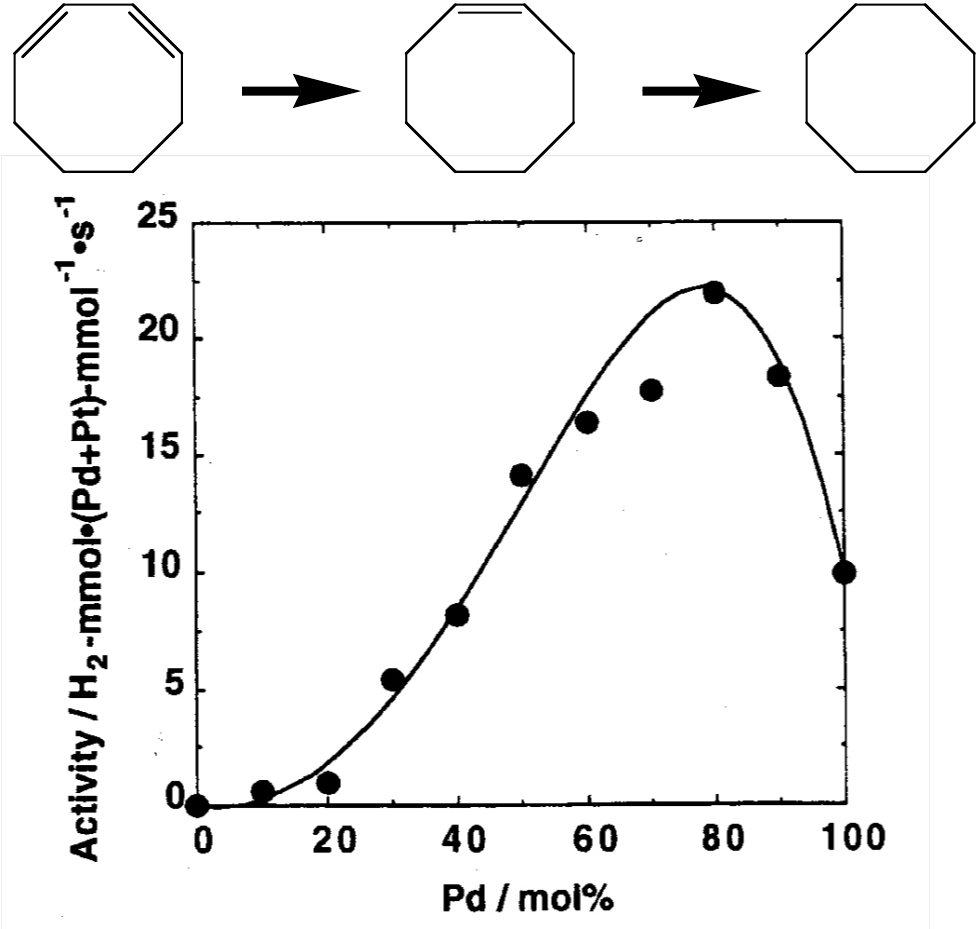
The chart below plots the reaction activity when the bimetallic nanoparticles are used as catalysts for hydrogenation.
The bimetallic nanoparticles were observed to not only be useful for the partial hydrogenation of 1,3-cyclooctadiene, which is a conjugated diene, but also to exhibit the highest activity level when the palladium content was 80% (Document 1 and 2). The electronic state of the metals might have changed because of the bimetal formation; however, we must understand the detailed structure of the nanoparticles.
The XPS results indicate that for bimetallic nanoparticles of palladium and platinum, palladium lies on the surface. Therefore, we examined the structure in detail using an X-ray absorption spectrum (Document 3 and 4). Among these methods, EXAFS spectroscopy is capable of identifying the types of scattering elements existing around the absorbing element, coordination number, and distance between atoms.
A computation using these factors indicates that the bimetallic nanoparticle contains 55 metallic atoms, and the shape and atomic arrangement of each of the atoms suggest that the nanoparticle has a palladium-platinum core-shell structure.
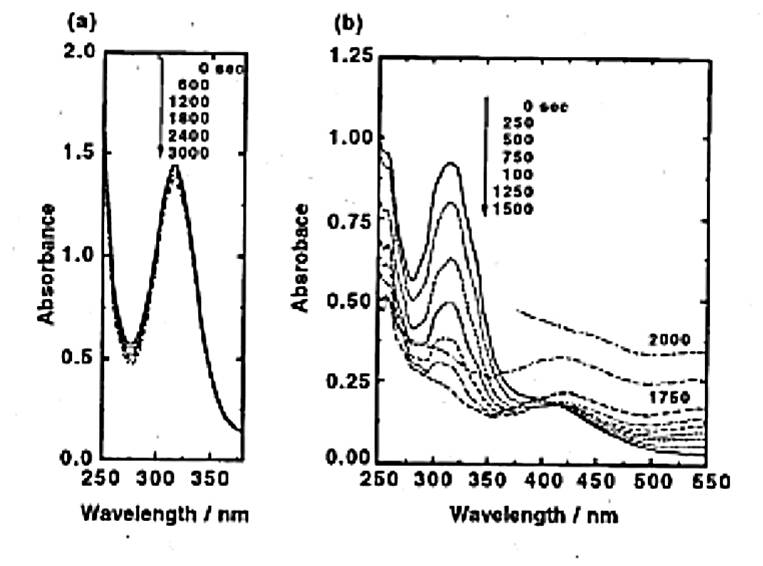
It is considered that the system that determines such core-shell structures can be explained by the difference in the reduction order that results from the difference in redox potential and the difference in the interactive force between the high polymer and metal. To determine the difference in the redox potential, we will examine variations at the time of palladium-gold reduction using UV-vis absorption spectra, in which the ion peaks can be easily identified (Document 5 and 6).
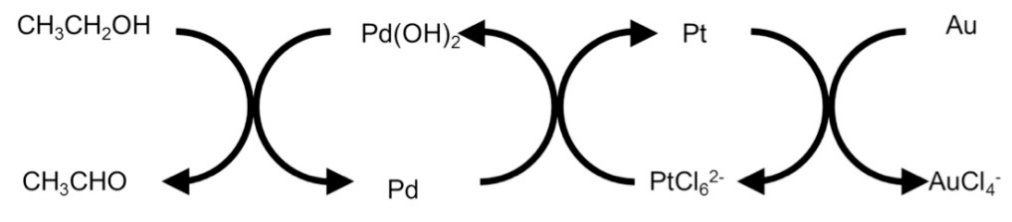
The figure shows that there is no variation in the peak of palladium until the reduction of gold is almost complete. This finding can be explained by the difference in the redox potential. However, another important factor is the “geared cycle.” The geared cycle refers to the mechanism in which the oxidation-reduction reaction seemingly proceeds like the meshing of gears. The case for the palladium-gold combination is illustrated below.
It has been observed, as explained above, that by alcohol reduction, core-shell bimetallic nanoparticles can be obtained.
In addition, we recently began the synthesis of octahedral particles (single crystal) with a consistent particle size and are making continuing efforts to examine their behavior. We are also designing and synthesizing fine particles and believe that the plasmon absorption effect can be applied in many areas.
Published Papers
- Naoki Toshima, Kakuta Kushihashi, Tetsu Yonezawa, and Hidefumi Hirai, “Colloidal Dispersions of Palladium-Platinum Bimetallic Clusters Protected by Polymers. Preparation and Application to Catalysis”,Chemistry Letters, 1989(10), 1769-1772.
- Naoki Toshima, Tetsu Yonezawa, and Kakuta Kushihashi, “Polymer-Protected Palladium-Platinum Bimetallic Clusters: Preparation, Catalytic Properties and Structural Considerations”, Journal of Chemical Society, Faraday Transactions, 89(14), 2537-2543 (1993).,
- Naoki Toshima, Tetsu Yonezawa, Masafumi Harada, Kiyotaka Asakura, and Yasuhiro Iwasawa, “The Polymer-Protected Pd-Pt Bimetallic Clusters Having Catalytic Activity for Selective Hydrogenation of Diene. Preparation and EXAFS Investigation on the Structure”, Chemistry Letters, 1990(5), 815-818.
- Naoki Toshima, Masafumi Harada, Tetsu Yonezawa, Kakuta Kushihashi, and Kiyotaka Asakura, “Structural Analysis of Polymer-Protected Pd/Pt Bimetallic Clusters as Dispersed Catalysts by Using Extended X-ray Absorption Fine Structure Spectroscopy”, The Journal of Physical Chemistry, 95(20), 7448-7453 (1991).
- Tetsu Yonezawa and Naoki Toshima, “Mechanistic Consideration of Formation of Polymer-protected Nanoscopic Bimetallic Clusters”,Journal of Chemical Society, Faraday Transactions, 91(22), 4111-4119 (1995).
- Naoki Toshima and Tetsu Yonezawa, “Bimetallic nanoparticles novel materials for chemical and physical applications”, New Journal of Chemistry, 1998(11), 1179-1201.
- Tetsu Yonezawa and Naoki Toshima, “Polymer- and Micelle-Protected Gold/Platinum Bimetallic Systems. Preparation, Application to Catalysis for Visible Light-Induced Hydrogen Evolution, and Analysis of Formation Process with Optical Methods”, Journal of Molecular Catalysis, 83, 167-181 (1993).